The press is talking about us!
A fine article published this weekend in Le Monde highlights the presentation given by Prof. Stephan Chabardès, neurosurgeon, head of the neurosurgery department at Grenoble Alpes University Hospital and head of the Clinatec hospital sector, at the WSSFN 2024 (World Congress of Stereotactic and Functional Neurosurgery) in Chicago on the use of intracranial photobiomodulation to stabilise Parkinson’s disease and the initial results of the associated clinical trial.
The Fonds Clinatec is particularly pleased and proud to have been able to contribute to this breakthrough from the outset, thanks to the support of its sponsors. Once again, Clinatec is opening up an unprecedented path towards a non-drug therapeutic solution that is likely to improve patients’ quality of life. Dr Laurent Hérault, Director of the Fonds Clinatec.
Link to the article in Le Monde
Read the abstract of the oral communication by Professor Chabardès
(in English)
RESTORING DOPAMINERGIC FUNCTION AFTER CHRONIC INTRACRANIAL PHOTOBIOMODULATION IN DE NOVO PARKINSON’S PATIENTS: A PROOF OF CONCEPT
Introduction
Recent studies in animal models of Parkinson’s disease (PD) have indicated that photobiomodulation (PBM) i.e., the use of red to near infrared light (λ=600-1300 nm) on body tissues, reduces the degeneration of midbrain dopaminergic neurons with favorable safety profile. Most manifestations of PD are related to the loss of dopaminergic neurons located in the substantia nigra pars compacta (SNc). Mitochondrial dysfunction is one of the key mechanisms leading to neurodegeneration in PD.
PBM restore mitochondrial function, resulting in an increase in adenosine triphosphate (ATP) energy production in cells and therefore can enhance survival of dopaminergic neurons and improve motor behaviour.
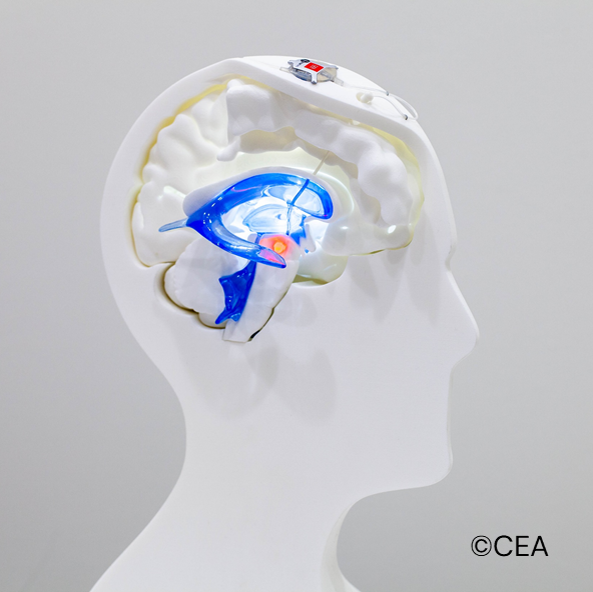
Here we report the first-in-man clinical and imaging data at 2 years f.up in 2 de novo PD patients fitted with a bespoke intracranial device delivering 670 nm light close to both SNc. We compared those data to 2 matched controls PD patients. The patients are part of a larger randomized, open-label case-control study (NCT04261569).
Methods
Four patients newly diagnosis with PD (less than 2 years), naive of any treatments were randomised to either PBM or control group. The probe of the PBM device was implanted into the floor of the 3rd ventricle, connected to a rechargeable battery. Clinical and PET imaging evaluation were compared to baseline and included among others MDS-UPDRS scale performed every 6 months and PET imaging every year using [11C]PE2I ligand.
Results
No abnormal feeling nor adverse events were noticed by the patients after chronic PBM. PBM Group: MDS-UPDRS scores part III improved at 24 months, from 38/132 to 23/132 and from 18/132 to 17/132 in patient#1 and #2 respectively, off drugs conditions. Control group: MDS-UPDRS scores part III deteriorated as expected from 20/132 to 33/132 and from 29/132 to 38/132 in patient#3 and #4 respectively, off drugs conditions.
With PBM, [11C]PE2I-PET at 12 (patient#1) and 18 months (patient#2) showed a remarkable increase of the [11C]PE2I BPND in the caudate nucleus, the nucleus accumbens and the putamen. The [11C]PE2I BPND decreased as expected in all basal ganglia in controls patients.
Conclusions
PBM is well tolerated and show promise for stabilzing motor symptoms and restoraring partially dopaminergic function